Hardness is the ability of a material to resist indentation and is measured by a variety of standardized tests that define the size, shape, and hardness of the indenter, and the load that is applied to create the indentation. Hardness values are then related to the length, width, or depth of the indentation. In hardened steels, hardness is most frequently measured with various loads on the Rockwell C scale (HRC), which uses a diamond cone indenter, or the Vickers hardness test (HV or DPH), which uses a diamond pyramid indenter. The Rockwell C tests are valid only for measurements on higher-hardness materials and are not valid below 20 HRC. Vickers and Brinell (HB) hardness measurements are valid not only for the HRC range but also for softer materials such as tool steels in the annealed condition.
High hardness and microstructures that have high hardness are the major objectives of the final heat treatments applied to tool steels. Ideally, in plain carbon and low-alloy steels, the highest hardness is achieved by forming microstructures that consist entirely of martensite. A completely martensitic structure is unattainable, however, even if the transformation of austenite to nonmartensitic microstructures can be suppressed. This is due to the incorporation of second-phase particles, such as inclusions and carbide particles that are not dissolved during austenitizing. Also, as carbon content increases, the amount of retained austenite increases.
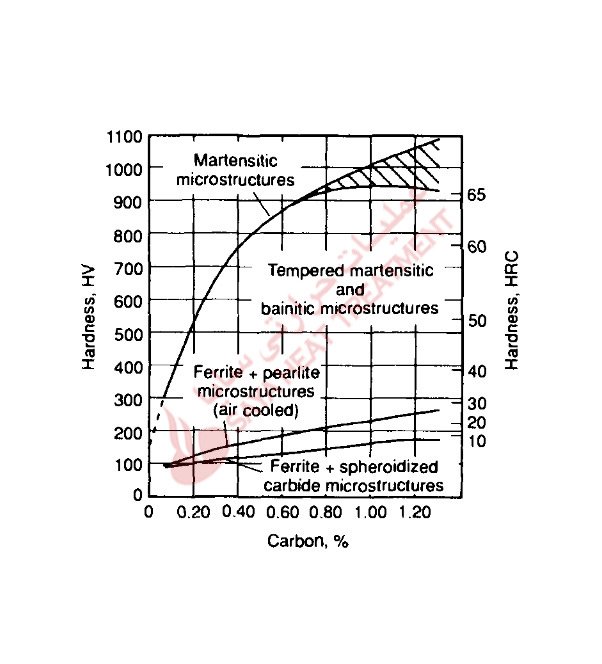
Figure 5-15 shows hardness as a function of carbon content for various microstructures that result from the transformation of austenite and various heat treatments of carbon steels. The highest hardness of any steel is associated with martensitic microstructures. The lowest hardness is associated with microstructures consisting of ferrite and spheroidized carbides produced by the annealing treatments described earlier. If the austenite transforms to microstructures such as ferrite, pearlite, or bainite, hardness will be lower than that of a martensitic microstructure. Also, as indicated in Fig. 5-15, tempered martensitic microstructures have lower hardness than as-quenched martensitic microstructures.
Hardness is related to chemical composition, crystal structure and perfection, and the size and distribution of the various phases that make up the microstructure. High hardness correlates with high resistance to slip and dislocation motion, high work-hardening rates, and high strengths. In martensite, the carbon-dependence of the high hardness of martensite is attributed to carbon atoms trapped in the octahedral interstitial sites of the martensitic crystal structure. Other contributing factors to the strength of martensite are the dislocation substructure of the martensite, dynamic interactions of carbon atoms with dislocations during strain hardening, and, in martensite tempered at low temperatures, the effect of fine transition carbide particles that precipitate from martensite supersaturated with carbon. Carbon, therefore, by its interaction with other structural elements of a martensitic microstructure, is the dominant factor controlling the strength of martensite. Substitutional alloying elements have a relatively small effect on the martensite strength.
Hardness, as discussed, is a measure of the strength of a microstructure and, in the context of heat-treated steels, is taken to be a measure of the strength of martensitic microstructures and the success of the hardening process. Hardenability refers to the ability of a given steel to form martensite, which in the context of heat-treated steels equates to the ability of a steel to form microstructures of the highest possible hardness. A comprehensive definition states: “Hardenability is the capacity of steel to transform partially or completely from austenite to some percentage of martensite at a given depth when cooled under some given conditions. In this regard, the widely used Bain and Grossmann hardenability system assumes full hardening with only 50% martensite. Finally, the definition indicates that cooling conditions influence martensite formation. The latter consideration relates factors such as quenching media, quenching effectiveness, and section size to the diffusion-controlled transformations of austenite that compete with martensite formation.
In order to compare the hardenabilities of steels as a function of composition, the Bain and Grossmann system removes the variations due to cooling in various media by establishing the concept of the ideal diameter. The ideal diameter, DI, of a steel is the diameter of a bar that hardens to 50% martensite in an ideal quench where the surface of the bar is assumed to cool instantly to the temperature of the quenching medium. Thus, the larger the ideal diameter, the higher the hardenability of the steel, independent of the technological factors that affect quenching.
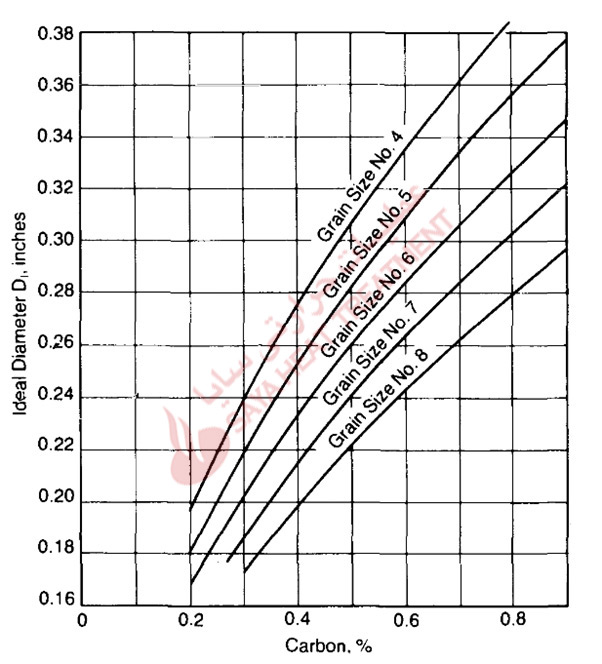
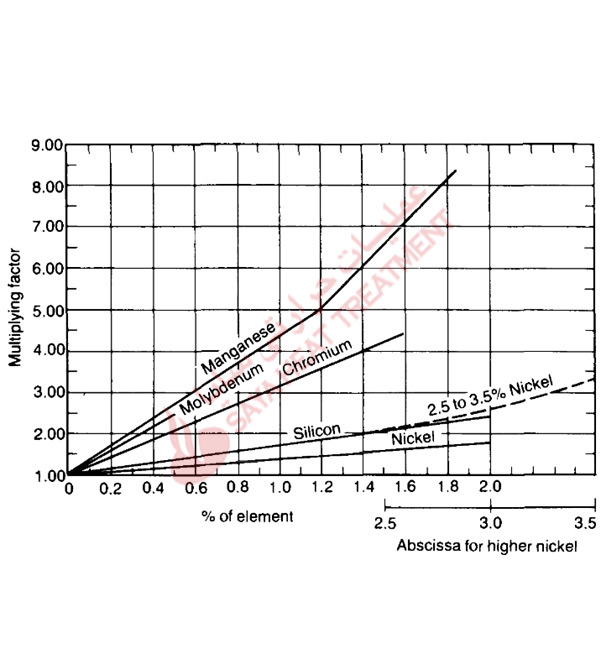
Hardenability is a function of carbon content, austenitic grain size, and alloy content, and Fig.5-16 and 5-17 show how these parameters are used to calculate ideal diameters. First, an ideal diameter based on steel carbon content and austenitic grain size is selected from Fig. 5-16, and then multiplying factors for the amounts of the various alloying elements are selected from Fig. 5-17. The ideal diameter from Fig. 5-16 is then multiplied by the factor for each alloying element to determine the final DI Critical diameters—the size of bars that will harden to 50% martensite in a given quenching medium—can then be determined for various quench severities. As the composition axes of Fig. 5-16 and 5-17 show, the Bain/Grossmann system for evaluating hardenability is applicable to medium-carbon steels with relatively low alloy contents.